Available Technologies
Lipid-Sensitive Endosomolytic Peptide for Cytosolic Antibody Delivery
Novel peptides that achieve efficient delivery of high-molecular-weight antibodies/proteins into cytoplasm
Background
One of the major obstacles to intracellular targeting by antibodies is their limited release from endosomes into the cytosol. Antibodies are taken up by cells via endoytosis, which is involves the physiological uptake of extracellular materials delivered into cells by encapsulation into vesicular compartments named endosomes.
Without their release from vesicular compartments into the cytosol, antibodies cannot interact with their target molecules, and may eventually be degraded in these compartments.
Applications
- Protein/antibody delivery
- Reagent for bioimaging/cell activity measurement
- Research tool for development of therapeutic antibodies/biopharmaceuticals
Description and Advantages
Researchers at Kyoto University have developed endosomolytic peptides that effectively deliver biomacromolecules, including antibodies, into the cytosol. The endosomolytic peptides are derived from the cationic and membrane-lytic spider venom peptide M-lycotoxin. By replacing leucine residue with glutamic acid (L17E) the delivery peptide specifically interacts with endosome membranes, breaking them down and releasing the antibodies.
- Facilitates the cytosolic release of endocytosed biomacromolecules
The effective release of antibodies (~150 kDa; Fig.2) and of small protein (FIg.3) into cytoplasm are verified. - Stimulates the physiological uptake of the cargo via the induction of micropinocytosis
- Allows antibodies to interact with target proteins, modulating their functions in cells
- L17E does not have a significant pH dependent membrane lytic activity
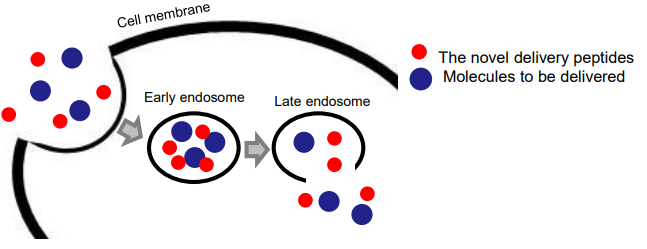
Fig. 1: Cytoplasmic protein delivery using the invented peptide
Successful intracellular protein delivery is achieved via the selective perturbation of endosomal membranes by the peptides.
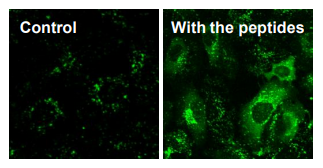
Fig. 2: Delivery of antibodies (~150 kDa)
Antibody delivery into HeLa cells observed with confocal microscope.

Fig. 3: Introduction of exogenous Cre recombinase (38 kDa) for gene recombination
Fluorescent signals ensure successful gene recombination by Cre.
| Development Status |
・ Release of antibodies (150 kDa) into cytoplasm verified ・ Release of small proteins into cytoplasm verified |
|---|---|
| Intellectual Property |
|
| Offer | ・ License (Non-exclusive) ・ MTA for sample evaluation |
| Related Links | View PDFView in Japanese |
Have you found what you were looking for?
- Interested in a particular research activity
- Cannot find the information
- Have questions on how to utilize research results
Feel free to contact us and get answers to your questions.
Inquiry- TLO-KYOTO
- Available Technologies
- Lipid-Sensitive Endosomolytic Peptide for Cytosolic Antibody Delivery
3rd Floor, International Science
Innovation Building, Kyoto University
Yoshidahonmachi, Sakyo-ku, Kyoto
606-8501 JAPAN


